
ReEvaluating antiretroviral Drug cOncentrations and Side Effects inindividuals living with HIV
WELCOME
We are a research team of people living with HIV, healthcare providers, researchers, and community members based in British Columbia, Canada, seeking to understand antiretroviral therapy, side effects and dosing in diverse groups of people living with HIV.
ReEvaluating antiretroviral Drug cOncentrations and Side Effects in Individuals Living with HIV (REDOSE)
REDOSE is a CIHR funded BCC3 substudy. This substudy takes on a new approach to integrate community engaged research principles into pharmacologic research, by uniting women and men living with HIV, researchers, clinicians and pharmacists to investigate the influences of age and sex on antiretroviral (ARV) concentrations and side effects.
Answering a Call From Community
The doses of medication are the same, you know, whether it’s a 300-lbs man or 120-lbs woman. So how is that affecting our bodies differently?
– Woman living with HIV
In March 2021, during the first BCC3 community advisory board meeting, a community member expressed concerns about women receiving the same doses of HIV medication (antiretrovirals, ARVs) as men. She urged our team to focus on this as a future research priority. Since then, we have heard similar concerns echoed from women across the province, alongside worries that ARV concentrations increase with older age.
The roots of these concerns trace back to historical development and testing of ARVs, which was predominantly conducted in cohorts of young men. Consequently, ARV doses have been universally applied, regardless of age and sex, based on studies that do not represent the diverse spectrum of individuals living with HIV. Age and sex play pivotal roles in how our bodies metabolize medications, yet our understanding of how ARVs interact with these factors remains alarmingly limited. It’s a gap our team is determined to bridge.
In the REDOSE study, we unite with community to explore the impact of older age and female sex on ARVs. We delve into these two key questions:
- How do age and sex impact ARV concentrations in the body?
- Are heightened ARV concentrations linked to increased side effects?
Through exploration of these questions, we seek to respond to the community’s call to action and challenge longstanding inequities in HIV treatment research.
Navigating the Study: A Two-Phase Approach
We expect that ARV concentrations and how these are impacted by age and sex will depend on the ARV regimen and when the sample is drawn after the last ARV dose taken. To optimize our understanding of ARV concentrations, we have designed two phases of our study:
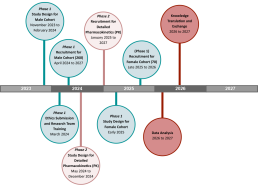
PHASE 1
In phase 1, our goal is to understand ARV concentrations and side effects broadly across several different ARV regimens in a diverse range of men and women living with HIV (260 men and 260 women). We will use previously collected data from the women in the BCC3 study, with some new female recruits in the later years (approx. 70 new recruits). We will also recruit 260 male participants from Oak Tree Clinic, St. Paul’s Hospital and other sites. Participants will be asked to complete one round of sample collection and a clinical survey, and a follow-up survey that can be done online independently or with a research assistant. We will then compare ARV concentrations and side effects between men and women and see if concentrations and side effects increase with older age.
PHASE 2
In phase 2, we will undertake a more detailed assessment of the most commonly prescribed ARV in British Columbia, called bictegravir (combined with other ARVs in a tablet called Biktarvy). To best understand how bictegravir concentrations differ by age and sex, we will measure drug concentrations at several time points within a 24-hour period after a dose of bictegravir is taken. This phase will take place with a smaller cohort of older women (60 years of age or more), younger women (less than 50 years of age), older men, and younger men with 20 participants in each group. By looking at several time points, we will be able to see all drug concentrations after a dose of ARV is taken (called a concentration curve). This will allow us to observe any differences between groups that might not have been picked up in Phase 1.
In both phases, participants must have been on an ARV regimen for at least two weeks, to allow the for the medication to settle in steady state, and they must be undetectable (viral load [VL] <40 copies/mL). Participants will be provided an honorarium for their time.
For more information on eligibility criteria and how to participate, please see the REDOSE participate page.
REDOSE Study Timeline
REDOSE Logo: The Story Behind the Design

Our REDOSE logo was designed by Leanne Flinton, UBC medical student, with guidance from our community consultant team. The pill shape represents our focus on ARVs with pink and blue colors representing our commitment to understand HIV medications in women and men living with HIV, respectively. We incorporate the red ribbon, a significant symbol of HIV activism, yet portray it gradually fading to symbolize the diminishing impact of HIV on the well-being and livelihoods of those it impacts. We hope through our work on better understanding ARVs, we can further optimize quality of life for people living with HIV by enhancing personalized care and minimizing drug related side effects.
Latest News and Events
Annual Women’s Health Research (WHRI) Symposium: March 8, 2024
Focusing on the science of the menopausal journey and its health impacts, the 9th Annual Women’s Health Research Symposium was held on March 8th, 2024. Our Co-op students, Camille Valbuena and Soleil Shraida, presented the first REDOSE poster, providing an overview of the study, our research methods, and our experience collaborating with community consultants on the design of study materials and surveys.
Kudos to our amazing team of researchers, students, and community consultants!







