THE SCIENCE BEHIND THE REDOSE STUDY
Here we provide detailed scientific background of the potential influence of sex and age on antiretroviral (ARV) concentrations in the body, and its impacts on the side effects experienced by people living with HIV
HIV is a lifelong condition that can be effectively managed with regimented use of HIV medications, known as antiretrovirals (ARVs)1, supporting people living with HIV to live longer lives2. Considering that these medications must be taken daily for decades, it is important to consider how these medications are absorbed, distributed, metabolized and eliminated in the body (processes called “pharmacokinetics”) over the years of usage. These pharmacokinetic processes changes with age3, and differs between sexes4. The pharmacokinetics of ARVs can determine the medication concentration in the body, and consequently, have an impact on side effects5.
Pharmacokinetics
Pharmacokinetics (PK) refers to the processes of how the body absorbs, distributes, metabolizes, and eliminates medications6. In order to gain a better understanding of how each of these become important in the processing of medications, it is helpful to know what these activities are.
Absorption
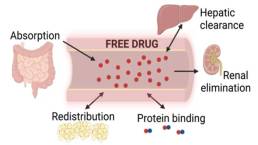
Once the medication is administered or consumed, absorption is how fast and how much will enter the bloodstream6. For medications taken orally, this will take place along the gastrointestinal (GI) tract, specifically in the small intestines (Figure 1), where the drug will cross the walls of the GI tract and into the bloodstream7.
Distribution
When the drug is absorbed into the blood circulation, it will travel through the bloodstream toward organs where it will perform its function (Figure 1). During its travels, there are molecules (proteins) that may bind to the drug and assist in its transport to the appropriate action sites in the body (Figure 1). The drug may also be deposited in other tissues of the body (muscles, fat, etc) that are not the direct site of action of the drug8.
Metabolism
Before the drug is active, it often needs to be chemically modified in the body, and transformed into different products that can further react in the body or that can be removed from the body. The primary organ responsible for metabolizing drugs is the liver9 (hepatic clearance; Figure 1).
Elimination
When a drug is then ready to be removed from the body, the drug will be excreted through a process called elimination. One primary organ responsible for this process is the kidney10 (renal elimination; Figure 1).
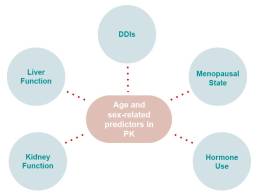
In each of these processes, any event that can change organ function6,9,10, alter the circulatory system6, or modify the body’s make-up of muscle and fat can change the pharmacokinetics11. Sex hormones12 and older age3 are two factors that are important to these networks of activities. In addition, one medication can sometimes interfere with the pharmacokinetic processing of another medication (a term called “drug-drug-interactions”, DDIs)13.
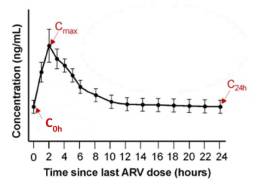
One way to investigate pharmacokinetics is by looking at drug concentrations at multiple timepoints after a dose of medication is taken. This allows us to create a concentration curve (Figure 3), or curve that demonstrates how much drug is in the body at different times. In a concentration curve, drug concentrations in the body often spike up shortly after taking a dose of medication, then slowly decline as time goes on. These curves can be separated by factors of age or sex and bring to light the differences in concentrations between groups, and where these differences are most likely to occur.
Effects of Age and Sex
Key Points:
- There are age-related physiologic changes that are known to alter the pharmacokinetics.
- There are sex-specific differences that change pharmacokinetics.
- Observational studies have shown that elderly individuals and women have a higher frequency of ARV side effects.
As people advance in age, their body water composition, body fat, and body mass changes, influencing the distribution of the circulating drug. In addition, they also have changes to their liver and kidney function13. These processes also differ by sex14. What’s more is that gender-associated behaviours and medications also impact drug concentration by altering body composition15, free circulating drug16, and metabolism17. Changes and differences like these can subsequently impact the ways the organs can filter, and eliminate substances (hepatic and renal clearance)9,10. Together, these changes can lead to higher drug concentrations in the body as people age and for women compared to men13.
These higher drug concentrations can accumulate and lead to drug toxicity, imparting negative effects on the individuals taking these medications5. Previous studies observed that this drug toxicity can place older people at higher risk to experience more ARV-related side effects, with one study suggesting that one potential side effect is a rapid decline of kidney function in these individuals13. For women, these side effects have manifested as more rashes18, weight gain19, sleep/mood effects20, and more. These negative effects subsequently decrease quality of life21, increase comorbid conditions22-23, and result in higher drug discontinuation rates24.
Knowing the effects of sex and age on pharmacokinetics is essential for us to understand what can be done to prevent these negative effects and improve quality of life.
Samples
What We Hope to Learn from the REDOSE Study Samples:
The REDOSE study combines community engaged research principles into pharmacologic research to investigate of the influences of age and sex on ARV concentrations and side effects in individuals living with HIV. Our samples and questionnaires give us important insights into the general and HIV-related health of women and men. See below to learn more about what each sample can teach us.
BLOOD SAMPLE
44 ml (3 tablespoons) of blood will be collected. HIV-related health information will be gathered from your clinical record, if available.
Your biospecimens (samples), collected as part of this study, will be tested for the following:
- drug concentrations of antiretrovirals;
- some common chemistry and hematology tests, including blood counts, kidney and liver function;
URINE SAMPLE
A urine sample will be collected to test for the level of a certain protein (called albumin) in the urine. This will tell us about your kidney health.
MOUTH SWAB (OPTIONAL)
Two mouth swabs will be collected, these will be used to test for cellular markers of health in the cheek cells collected. The mouth swabs will also tell us some of what we would learn from blood to help us collect data in a less invasive way.
RECTAL SWAB (OPTIONAL)
We are requesting two rectal swabs at this visit – to be self-collected by you. Your biospecimens will have testing done in the biological science area called “omics”. This is a field of study that looks at the group classification and measurement of pools of biological molecules that translate into the structure, function, and dynamics of an organism or organisms. This testing will be used to investigate links between the rectal microbiome (as a surrogate for a stool/gut sample) and the other health markers we are looking at in this study.
References
- (1) Deeks, S. G.; Lewin, S. R.; Havlir, D. V. The end of AIDS: HIV infection as a chronic disease. Lancet 2013, 382 (9903), 1525-1533. DOI: 10.1016/S0140-6736(13)61809-7.
- (2) UNAIDS. HIV and aging – A special supplement to the UNAIDS report on the global AIDS epidemic 2013. https://reliefweb.int/report/world/hiv-and-aging-specialsupplementunaids-report-global-aids-epidemic-2013. Accessed November 15, 2019.
- (3) Klotz, U. Pharmacokinetics and drug metabolism in the elderly. Drug Metab Rev 2009, 41 (2), 67-76. DOI: 10.1080/03602530902722679.
- (4) Stader, F.; Marzolini, C. Sex-related pharmacokinetic differences with aging. Eur Geriatr Med 2022, 13 (3), 559-565. DOI: 10.1007/s41999-021-00587-0.
- (5) Han, J. H.; Chen, A.; Vasilevskis, E. E.; Schnelle, J. F.; Ely, E. W.; Chandrasekhar, R.; Morrison, R. D.; Ryan, T. P.; Daniels, J. S.; Sutherland, J. J.; et al. Supratherapeutic Psychotropic Drug Levels in the Emergency Department and Their Association with Delirium Duration: A Preliminary Study. J Am Geriatr Soc 2019, 67 (11), 2387-2392. DOI: 10.1111/jgs.16156.
- (6) Grogan, S. (2023, July 30). Pharmacokinetics. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK557744/. Accessed March 28, 2024.
- (7) Vertzoni, M., Augustijns, P., Grimm, M., Koziolek, M., Lemmens, G., Parrott, N., Pentafragka, C., Reppas, C., Rubbens, J., Van Den Αbeele, J., Vanuytsel, T., Weitschies, W., & Wilson, C.G. (2019). Impact of regional differences along the gastrointestinal tract of healthy adults on oral drug absorption: An ungap review. European Journal of Pharmaceutical Sciences, 134, 153–175. https://doi.org/10.1016/j.ejps.2019.04.013
- (8) Le, J. (2024, March 18). Drug distribution to tissues – clinical pharmacology. Merck Manuals Professional Edition. https://www.merckmanuals.com/en-ca/professional/clinical-pharmacology/pharmacokinetics/drug-distribution-to-tissues. Accessed March 28, 2024.
- (9) Susa, S. T. (2023, August 17). Drug metabolism. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK442023/. Accessed March 28, 2024.
- (10) Garza, A. Z. (2023, July 4). Drug elimination. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK547662/. Accessed March 28, 2024.
- (11) Schwartz, J. B. The current state of knowledge on age, sex, and their interactions on clinical pharmacology. Clin Pharmacol Ther 2007, 82 (1), 87-96. DOI: 10.1038/sj.clpt.6100226.
- (12) Zucker, I.; Prendergast, B. J. Sex differences in pharmacokinetics predict adverse drug reactions in women. Biol Sex Differ 2020, 11 (1), 32. DOI: 10.1186/s13293-020-00308-5.
- (13) Klotz, U. Pharmacokinetics and drug metabolism in the elderly. Drug Metab Rev 2009, 41 (2), 67-76. DOI: 10.1080/03602530902722679.
- (14) Stader, F.; Marzolini, C. Sex-related pharmacokinetic differences with aging. Eur Geriatr Med 2022, 13 (3), 559-565. DOI: 10.1007/s41999-021-00587-0.
- (15) Ford, K.; Huggins, E.; Sheean, P. Characterising body composition and bone health in transgender individuals receiving gender-affirming hormone therapy. J Hum Nutr Diet 2022, 35 (6), 1105-1114. DOI: 10.1111/jhn.13027.
- (16) Badowski, M. E.; Britt, N.; Huesgen, E. C.; Lewis, M. M.; Miller, M. M.; Nowak, K.; Sherman, E.; Smith, R. O. Pharmacotherapy considerations in transgender individuals living with human immunodeficiency virus. Pharmacotherapy 2021, 41 (3), 299-314. DOI: 10.1002/phar.2499.
- (17) Le, A.; Huang, K. J.; Cirrincione, L. R. Regulation of drug-metabolizing enzymes by sex-related hormones: clinical implications for transgender medicine. Trends Pharmacol Sci 2022, 43(7), 582-592. DOI: 10.1016/j.tips.2022.03.006.
- (18) Wong, K. H.; Chan, K. C.; Lee, S. S. Sex differences in nevirapine rash. Clin Infect Dis 2001, 33 (12), 2096-2098. DOI: 10.1086/324088.
- (19) Lake, J. E.; Wu, K.; Bares, S. H.; Debroy, P.; Godfrey, C.; Koethe, J. R.; McComsey, G. A.; Palella, F. J.; Tassiopoulos, K.; Erlandson, K. M. Risk Factors for Weight Gain Following Switch to Integrase Inhibitor-Based Antiretroviral Therapy. Clin Infect Dis 2020, 71 (9), e471-e477. DOI: 10.1093/cid/ciaa177.
- (20) Hoffmann, C.; Welz, T.; Sabranski, M.; Kolb, M.; Wolf, E.; Stellbrink, H. J.; Wyen, C. Higher rates of neuropsychiatric adverse events leading to dolutegravir discontinuation in women and older patients. HIV Med 2017, 18 (1), 56-63. DOI: 10.1111/hiv.12468.
- (21) Tramarin, A.; Parise, N.; Campostrini, S.; Yin, D. D.; Postma, M. J.; Lyu, R.; Grisetti, R.; Capetti, A.; Cattelan, A. M.; Di Toro, M. T.; et al. Association between diarrhea and quality of life in HIV-infected patients receiving highly active antiretroviral therapy. Qual Life Res 2004, 13 (1), 243-250. DOI: 10.1023/B:QURE.0000015282.24774.36.
- (22) O’Halloran, J. A.; Sahrmann, J.; Parra-Rodriguez, L.; Vo, D. T.; Butler, A. M.; Olsen, M. A.; Powderly, W. G. Integrase Strand Transfer Inhibitors Are Associated With Incident Diabetes Mellitus in People With Human Immunodeficiency Virus. Clin Infect Dis 2022, 75 (12), 2060-2065. DOI: 10.1093/cid/ciac355.
- (23) Kuo, P. H.; Sun, H. Y.; Chuang, Y. C.; Wu, P. Y.; Liu, W. C.; Hung, C. C. Weight gain and dyslipidemia among virally suppressed HIV-positive patients switching to co-formulated elvitegravir/cobicistat/emtricitabine/tenofovir alafenamide. Int J Infect Dis 2020, 92, 71-77. DOI: 10.1016/j.ijid.2019.12.029.
- (24) Kempf, M. C.; Pisu, M.; Dumcheva, A.; Westfall, A. O.; Kilby, J. M.; Saag, M. S. Gender differences in discontinuation of antiretroviral treatment regimens. J Acquir Immune Defic Syndr2009, 52 (3), 336-341. DOI: 10.1097/QAI.0b013e3181b628be.